Data Entry & Reporting
Managing patient records in αlpha is easy. Reported and unreported patient data are both managed from the Patients screen and new patient data can be entered manually or imported from a file.
Unreported patient data is grouped into named batches to help organise the screening program. Screening reports are made for each patient in a batch and these reports can be printed or exported in a number of formats. Once a report has been issued it is stored in the αlpha database from where it can reprinted or, if necessary, corrected and a modified report issued.
Data entry
Data entry in αlpha has been designed to be clear, fast and accurate. The main features of the αlpha data entry screen are:
- All prompts appear on a single screen and the same screen is used for all screening tests.
- A preview can be made of the screening report for the current patient
- The data entry screen layout can be modified to the user's requirements
- Default settings can be specified for frequently used fields
- Codes can be assigned to doctors, sonographers and addresses. These codes can then be used to speed up data entry.
- Searching for specified terms is possible within batches
The screen below show a data entry screen. This data entry screen has been configured to allow entry of first trimester and second trimester markers for both Integrated and non-integrated tests.
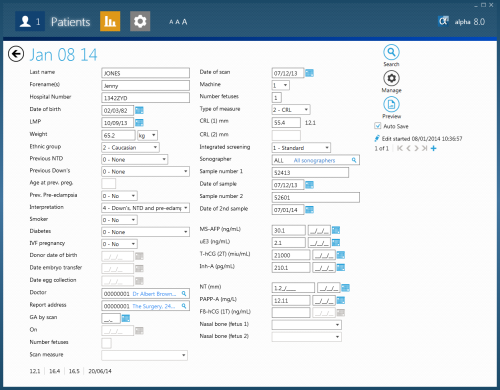
Test interpretation and risk estimation
alpha estimates a woman's risk of having a Down's syndrome pregnancy by determining the age-specific risk of Down's syndrome, and modifying it in the light of the screening marker levels, using a multivariate log-Gaussian model derived from published parameters. A similar method is used to estimate the risk of having a pregnancy affected with trisomy 18, trisomy 13 or SLOS.
The log-Gaussian model is used to generate a Likelihood Ratio (LR), which is used to modify the age-specific risk, according to the levels of the different screening markers, as follows:-
Test specific risk (as an odds ratio) = LR x Age specific risk (as an odds ratio)
The marker levels are expressed as multiples of the normal median (MoM values), thereby allowing for changes in the normal median with gestational age, and for systematic differences between laboratories and between assay reagents.
The MoM values may be adjusted to allow for other factors which affect the normal median value, such as maternal weight, ethnic group, insulin-dependent diabetes, smoking, in-vitro fertilization and multiple pregnancy.
By using MoM values in this way, alpha is independent of the assay reagents used. The software is designed to allow you to monitor the normal medians, and to change them if necessary; for example, to correct for drift in the normal medians, or if you decide to change the assay reagents used.
Reporting
Our philosophy is that patient reports should be simple, informative and clear. Reports are stored in a database, allowing you to reprint a copy of any report. If changes or additions are made to a report, αlpha retains both the original report and all modified versions, so a full report history and audit trail is available for every woman screened.
αlpha provides screening interpretations for Down's syndrome, trisomy 18, trisomy 13, open neural tube defects and pre-eclampsia and provides the facility for identifying pregnancies with a high risk of SLOS.
The data entry screen and reports can be shown in 16 languages (English, German, Italian, French, Greek, Spanish, Portuguese, Turkish, Russian, Czech, Slovak, Vietnamese, Romanian, Chinese, Polish and Hebrew)
The figure below shows a screening report for an Integrated test with screening interpretations for Down's syndrome, neural tube defects and pre-eclampsia. The trisomy 18 and 13 risks are shown as incidental results of screening for Down's syndrome.
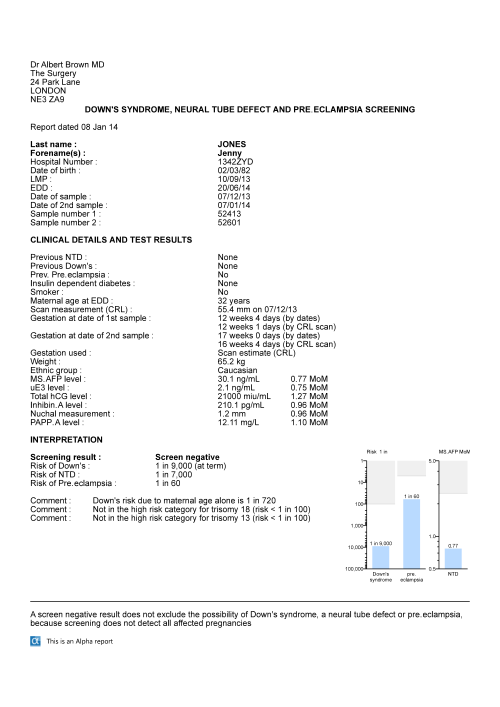
The figure below shows a screening report for an Integrated test with screening interpretations for Down's syndrome, trisomy 18, trisomy 13, neural tube defects and pre-eclampsia. The report header has been designed by the user using the facilities provided in Alpha.

Multi-user version
αlpha is available in a basic single-user configuration, or with multi-user access. Benefits of multi-user access include:-
- Increased throughput - several users can enter data simultaneously at different workstations
- Greater convenience - reports can be viewed or printed from any location on the network
More reporting features
Other reporting features in αlpha include:
Screening tests
- Provides estimates of risk for Down’s syndrome between 10 and 22 weeks and open neural tube defects (NTD) between 15 and 22 weeks using all recognized screening tests.
- Allows screening for Down’s syndrome, trisomy 18 and trisomy 13 in the first trimester using serum markers and nuchal translucency, either alone or in combination
- Interprets Integrated test results for safe and effective Down's syndrome screening.
- Interpretations can be made using a sequential testing protocol, with very high risk women having a first trimester screening result and nearly all the remaining women proceeding to have an Integrated test.
- Single risk of Down's syndrome, trisomy 18 or trisomy 13 or single risk of trisomy 18 or trisomy 13 can be reported
- Can implement a DNA reflex screening protocol
- Allows user to specify separate screening cut-off levels for different screening tests
- Provides diagnosis of open neural tube defects using amniotic fluid Alpha-fetoprotein (AF-AFP) and Acetylcholinesterase (AcheE) results
Risk calculation
- Allows for maternal age, gestational age, ultrasound measurements, ethnic group, maternal weight, past history of NTD and Down’s syndrome
- Allows for differences in marker levels and maternal weight in up to seven ethnic groups, either using separate medians and weight adjustments, or using correction factors
- Normal medians for the screening markers are derived from local data
- Separate median equations for gestational age estimated by dates and scan, if desired.
- Calculates gestational age from various fetal measurements including crown-rump length (CRL), biparietal diameter (BPD), abdominal circumference (AC) and head circumference (HC).
- Adjusts risk estimate for the age at which a previous pregnancy was affected with Down’s syndrome
- Nuchal translucency measurements adjusted for CRL
- αlpha offers the option of sonographer specific nuchal translucency medians to allow for systematic differences between measurements made by different sonographers. This simple measure provides a useful improvement in screening performance
- User defined option to report Down’s syndrome, trisomy 18 and trisomy 13 risk at term, or early second trimester or late first trimester
- Option to include the result of an ultrasound nasal bone examination
- Option to include categorical assessment of ductus venosus blood flow
- αlpha adjusts serum marker levels in In vitro fertilization (IVF) pregnancies to correct the otherwise high false positive rate
- In IVF pregnancies the date of egg collection and, for donated eggs, the donor date of birth can be specified.
- Screening results given for twin pregnancies and insulin dependent diabetic pregnancies
- Uses NT, chorionicity, nasal bone status and serum markers in interpretation of twin pregnancies. An option is provided to show fetus specific risks in a twin pregnancy if only NT measurements are provided.
- In twin pregnancies, gestational age can be derived from either the larger or the average of the two fetal measurements (CRL or HC).
- αlpha adjusts serum marker levels in smokers
- αlpha adjusts screening markers to avoid a high recurrent false positive rate
- αlpha can identify cases where a single marker has a very large influence on the risk estimate raising the possibility that the risk estimate may be incorrect. In these cases, αlpha will notify the user of the anomalous marker and give them the opportunity of removing it from the risk estimate in the screening report.
- Option to specify gestational age range to use for second trimester samples
- Repeat testing is best avoided, but if done, it requires an interpretation which takes into account the previous result; αlpha does this
- Option to “cap” very high risk estimates or “trim” very low risk estimates
Reporting
- All reports can be exported in XPS or PDF format
- The risk estimate can be shown graphically on the screening reports
- Custom designed reports available on request
- Option to design headers on reports which so that pre-printed headed paper is not required. The headers can contain image files, text and simple graphics